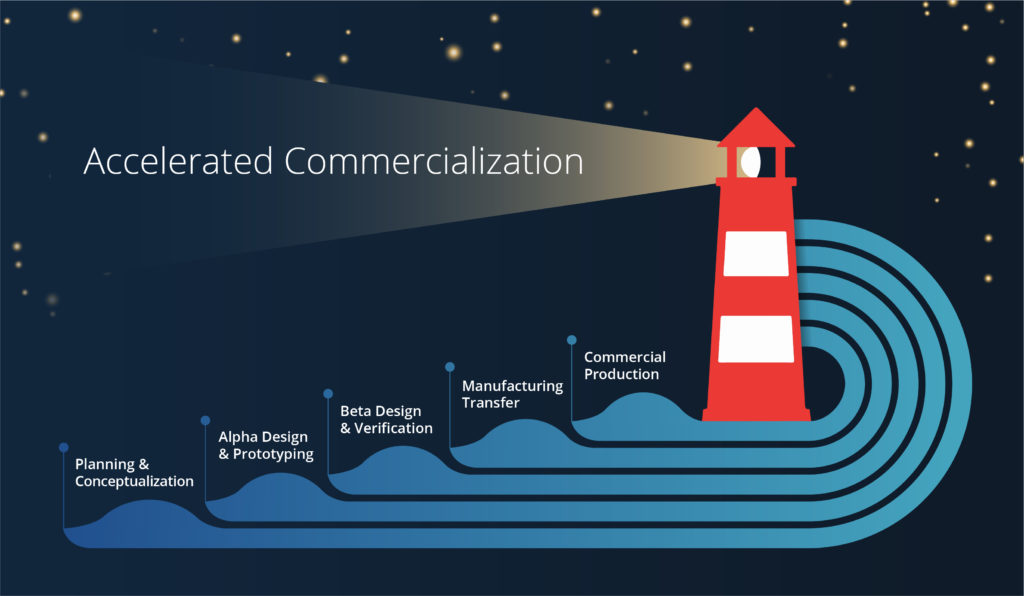
LIGHTHOUSE IMAGING PRODUCT DEVELOPMENT PROCESS
Lighthouse utilizes a traditional phase/gate process that takes medical devices from concept through commercialization. We understand the importance of design control and risk management activities and incorporate these into our rigorous product development process. Our ISO 13485:2016 certification is a testament to this rigor and our commitment to product development excellence.
Each product development project is assigned an experienced team including a project manager and systems engineer to lead the project through the product development process. Mechanical, Electrical, Optical, Manufacturing and Quality engineering resources are also assigned depending on the project needs. Our proficient engineering team has experience with miniature visualization systems, optical systems for surgical robots, chip-on-tip endoscopic systems, optical lens design and 3D systems.
Our product development process accelerates the overall timeline by minimizing design changes in the later stages of development. With medical device development, design changes become increasingly costly (time and money) closer to commercialization. By investing in a planning phase up front and holding regular design reviews we minimize these design changes.
Our product development process also incorporates design for manufacturability from the start. We understand that cost and product performance are both important to our clients. We consider cost implications with every major design decision, educate our clients on these implications and make decisions collaboratively. This avoids unnecessary surprises and ensures that our client’s needs are met.
The 5 Optical Design Parameters to consider for Chip on Tip Endoscopes.
Defining optical design specifications for custom chip-on-tip endoscopes can be a daunting task. In optics, the critical specifications are literally invisible, and it is common that parameters critical to the success of the device are overlooked.
