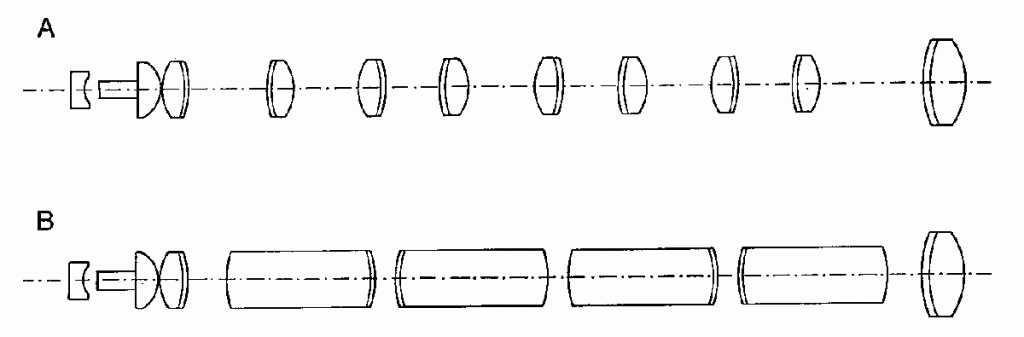
Designing Optical Device Products for OEM Manufacturability
From day one, Lighthouse Imaging is focused on designing optical systems and modules for manufacturability for the most demanding OEM partners. There’s a heavy emphasis on the planning phase to define the optical product requirements and the product development strategy as defined and influenced by the regulatory strategy. Additionally, there is a very focused effort on defining the product requirements, not only meeting visualization performance specifications but just as important, the product cost. This allows us to then go into the development phase with an effective, efficient process, understanding the performance and economic objectives.
By working with Lighthouse from the onset of a medical optics project, and not just from when a product is “deemed ready,” clients can reduce the timeline for commercialization as they are developing a product for manufacturing, not just a product to meet performance criteria.
This ability to add experience to the early stage planning and strategy really positions medical device OEM’s to be most successful in meeting their end goals. Specifically, in the early periods of planning, it is crucial to define objectives, outline deliverables, and discuss measurable results. By thoroughly understanding the client’s end goals, Lighthouse can attack the project and produce the optimum outcomes for performance, unit cost and time to market. Because Lighthouse has extensive experience in all areas of optical design and manufacturing, an industry focus, and a thorough understanding of the client’s end goals, we are able to optimally position their partners for success.
Our team takes the concept from paper to a commercial medical device, carrying it through a regulatory compliant development process, supporting the customer throughout, and ultimately, manufacturing the optical system at a targeted cost. This provides the innovator with a viable intellectual property they can leverage to add value to their company. In the design, development, and manufacturing process, the communication and cohesiveness working with Lighthouse Imaging continues to set us apart as a preferred contract manufacturing partner.
Our Commitment to Developing World Class Optical Manufacturing & Quality Visualization Systems
The management team at Lighthouse Imaging is committed to continually improving our manufacturing and quality systems. Our quality management system has received both ISO 9001 and 13845 certification and our facility is an FDA Registered Facility, compliant to CFR 820 Medical Device regulations.
Manufacturing Systems
- Advanced ERP Systems
- Allows for the control of all materials from procurement throughout manufacturing while providing material traceability for the life of the device.
- Bar coding system enables tracking of serialized devices
- New investment in an EPDM system
- Electronic document control system
- Provides version control and historical traceability of all revisions for both Drawings and Documents
- Utilize a full Project Management Software suite
Material Control
- Rigorous Incoming Inspection
- Inspection plans are developed in accordance with risk factors developed during the design phase and in accordance with industry standards.
- Materials are received, inspected and released according to inspection procedures and drawings
- In accordance with cGMP, we have full lot control and traceability on all materials, processes, operators.
- Done through lot number assignment, serial number assignment, work order tracking and color-coded labeling
Supply Chain
- Decades of development of Precision European & Domestic optics vendors for advanced components
- Network of Asian sources of optical components used in production for over a decade
- Broad range of PCB and electronics suppliers, distributors both domestically and internationally.
- Mechanical and machined components with quick turnaround from domestic vendors in Maine, Massachusetts and NY are used for majority of production
- Cost reduction opportunity available using overseas vendors for components and subassemblies
- All suppliers are monitored for performance under our Quality Management System.
Pathway to Commercialization – Whitepaper
There will be many challenges that are experienced along the development path that will require tradeoffs in product features versus unit costs. Constant consideration of the objectives defined in the ideation stage are imperative for effective commercialization. This evaluation should span the entire development process.
