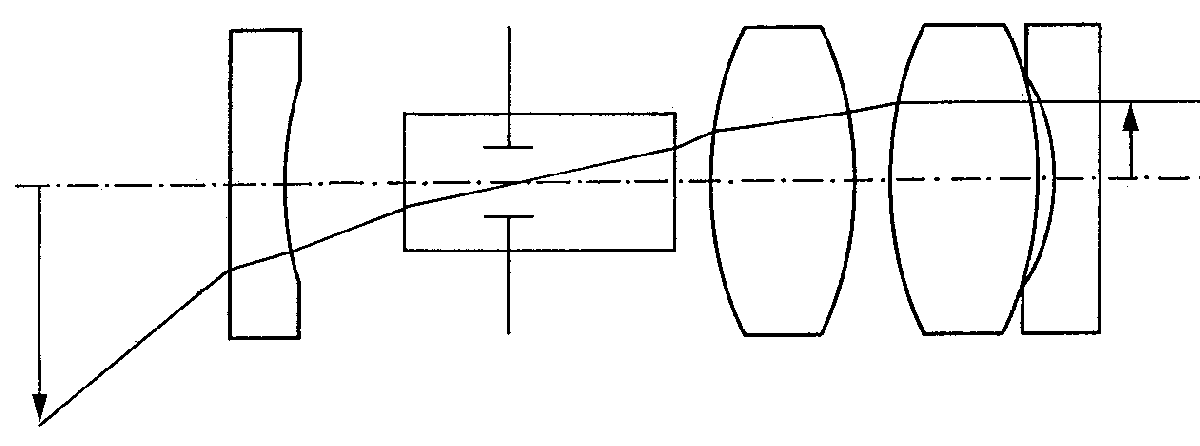Dennis C. Leiner
Lighthouse Imaging Corporation
769 Roosevelt Trail, Suite 9
Windham, ME 04062
ABSTRACT
The optical design and construction of flexible, gradient-index, and conventional endoscopes are described in this paper. Lens and prism components used in medical endoscopic instrumentation are discussed. Emphasis is given to the practical considerations of manufacturing these systems that are unique to their small size and use in a hospital environment.
1. INTRODUCTION
Escalating health care costs in the last two decades have helped promote a new form of treatment known as least-invasive surgery. Diagnosis-related rather than treatment-related insurance reimbursements have encouraged less traumatic surgical procedures requiring shorter hospital stays and faster recuperation. Patients have become more educated as to the medical options available for treatments and are demanding all that technology has available to minimize their pain and recovery time. Endoscopic procedures often play a pivotal role in least-invasive surgery and have been encouraged by insurance companies and hospital administrators who are anxious to reduce treatment costs and provide the most effective choices obtainable.
As an example, a common orthopedic surgery is the meniscectomy, in which torn cartilage is removed from the knee. Until 1974 all meniscectomies done in the United States were open procedures requiring a large incision, a full leg cast, and a recovery period of several months. Arthroscopic meniscectomies currently performed require only a few puncture wounds in the area of the knee for insertion of the 4 mm diameter arthroscope and the instrumentation for irrigation and surgery. Patients begin physical therapy the same day as the surgery and can often be released the following day.
Rigid endoscopes can contain as many as 60 lens components for relaying the image from the inside of the body. Diameters of optical components can be 1 mm or less. Quantities sold by any one manufacturer range only between a few hundred and a few thousand endoscopes per year and production techniques have always contained an element of craft. Accelerating interest in the last decade in advanced endoscopes with superior brightness and resolution and smaller diameter have advanced the limits of technology in producing these lens systems cost-effectively. Most medical endoscopes are manufactured in Germany or Japan. Rising prices of foreign products and the desire to better control the product design, manufacture, and repair have created an insistence in bringing the manufacture of endoscopes into the United States.
2. HISTORY
Figure 1. History of the endoscope | |
| .> | |
| Nitze (1879) – | 7 mm Cystoscope |
| Burning platinum wire illumination | |
| Schindler (1936) – | Semi-flexible gastroscope |
| 48 lenses in spiral spring | |
| Hirchowitz (1958) – | Optical fiber gastroscope |
| Hopkins (1960) – | Rod lens patent filed |
| Nippon Sheet Glass (1968) – | Selfoc developed |
| Watanabe (1970) – | 1.7 mm gradient index endoscope |
The history of the use of miniature optics in endoscopy is outlined in Figure 1. The first endoscope using a miniature lens system was a cystoscope designed by Nitze with the help of an optician from Berlin and an instrument designer from Vienna.2 A gastroscope of similar design was built the following year. These first endoscopes incorporated a distal reflecting prism to incline the direction of view. In 1931, the first practical arthroscope was developed for knee surgery.3 The instrument was 3.5 mm in diameter and contained a right-angle reflecting prism at the distal tip.
Schindler introduced the first flexible endoscope with a German optical physicist named Wolf.2 The instrument was 77 cm long, the last 43 cm being made flexible by a steel spiral covered by rubber tubes with an outside diameter of 12 mm. Optical fiber was first used for flexible endoscope imaging in 1958 by Hirchowitz, Curtis, Peters, and Pollard.2 Rigid endoscope optics also improved in this time period with the introduction of the now well accepted rod lens designs of H. H. Hopkins.
2.1 Gradient-index Endoscope
The development of Selfoc® gradient-index rod lenses which are manufactured primarily for table-top copiers has proved to be of importance in the development of small diameter endoscopes. Only two years after the development of Selfoc, Watanabe in Japan built a 1.7 mm diameter gradient-index endoscope.3 The first gradient-index endoscopes were of limited use because of large amounts of chromatic aberration in the Selfoc material. In 1984, improved diffusion components in the Selfoc improved chromatic aberration to the extent that resolution is now comparable to many conventional small diameter (< 2 mm O.D.) endoscopes.
3. FIBEROPTIC ENDOSCOPE OPTICS
The first-order optics of a fiberoptic endoscope is straightforward. (See Figure 2.) An objective lens system is inserted into the body and produces an image of the desired internal structure onto one end of a 2-dimensional array of clad optical fibers.
Figure2 . Optical layout of a fiberoptic endoscope
These arrays are historically known as coherent fiber bundles but must of course be distinguished from our current reference to single-mode devises. Each fiber has a diameter on the order of 10 micrometers with a typical bundle containing several hundred thousand fibers.
Each fiber in the bundle effectively receives one pixel of information about the image which is relayed to the other end of the bundle to the outside of the body. Since the fibers are only fixed to each other at the ends, most of the length of the endoscope can be flexed to allow passage deep into the body. The image at the proximal, or observer’s side of the fiber bundle is viewed with a simple ocular that presents a magnified view to the eye.
3.1 Objective Design
Because the acceptance cone and output cone for the individual fibers is symmetrical about the axis, the chief ray is exactly telecentric at both the distal and proximal ends of the coherent bundle. This is an important consideration in designing an objective system that provides uniform illumination at the edge of the field. Aberration correction of the objective lens is also complicated by the presence of the fiber bundle relay in the optical train. With the possible exception of lateral chromatic aberration and distortion, none of the other aberrations present in the objective can be corrected by overcorrection of the ocular. In addition focal position must be adjusted independently at the distal end to maintain a sharp focus at the distal end of the fiber bundle. The patent literature contains many flexible endoscope objective system designs, some with as many as 20 spherical surfaces.4
The outside diameter of (a) many flexible endoscopes is on the order of a centimeter so that lens production by conventional methods can be employed. Smaller diameter flexible endoscopes are limited by the individual fiber diameter. The real challenge to the miniature lens designer and optician is the rigid endoscope, which uses lenses rather than fiber to relay the image to the outside of the body. Rigid endoscopes are preferred over flexible endoscopes if the medical procedure permits it because of greater resolution potential and the absence of a visible lattice structure.
4. GRADIENT-INDEX ENDOSCOPE OPTICS
The basic gradient-index endoscope consists of two different types of Selfoc material. These are called the objective lens and the relay lens. The radial refractive index gradient of the Selfoc material is designed to periodically focus an image along its length. The objective lens is much shorter than the relay lens and is bonded to the end of the relay lens. The objective lens focuses light from an object onto its rear face.
4.1 Optical Design
The entrance pupil of the endoscope is located at the front focal plane of the objective lens. The pupil is telecentric between the objective and relay lenses. The chief ray is also the extreme ray of the system. The natural aperture stop of the endoscope is the wall of the relay lens at a distance of one-quarter period of the relay lens from the back of the objective lens. Equivalent aperture stops are located at one-half period internals along the length of the relay lens.
Image positions in the relay lens are separated from the aperture stops by one-quarter period, the first image being at the interface of the objective lens and relay lens. The orientation of successive images is reversed. The length of the relay lens is adjusted for the desired endoscope length but must be an integral number of half-periods.
4.2 Brightness
The brightness of the image in the endoscope is ultimately determined by the optical invariant of the relay section, which is proportional to the numerical aperture and diameter of the relay lens. The theoretical invariant and thus the maximum brightness of gradient-index relays is larger than conventional systems of equal diameter because of the absence of glass-to-air refractions in gradient systems. The vignetting of a gradient-index endoscope is 100% when the relay lens walls are the aperture stop. The vignetting can be reduced if necessary by inserting a field stop into the system. An aperture stop will improve the uniformity of illumination over most of the field.
4.3 Field of view
Selfoc objective lenses currently available limit the field of view of the endoscope to about 55°. This is less than is desired since these small diameter endoscopes are used in very confined areas with only very small object distances possible. To increase the field of view a negative lens can be added to the tip of the endoscope.5 This allows rays from a larger object field angle to be accepted by the objective lens. When this field-widening lens is used, the chief ray is no longer parallel with the axis at the image plane, as in a normal gradient-index endoscope. Also the height of the image is smaller than the diameter of the optics, in contrast to a normal GRIN scope. In order to maximize the optical invariant and thus the light throughput of the system, it can be shown that the aperture stop and field stop must be separated by one-quarter period of the relay lens. This is equivalent to stating that the chief ray must be parallel with the axis at the image locations.
A convenient means of achieving this condition is by the insertion of a homogeneous glass spacer cylinder between the objective lens and relay lens. The objective lens thickness is decreased to where the chief ray is parallel to the axis, and the spacer thickness is adjusted such that an image falls on its rear face. This allows the chief ray to achieve its maximum height at the image and increases the optical invariant to the maximum permitted by the relay lens.
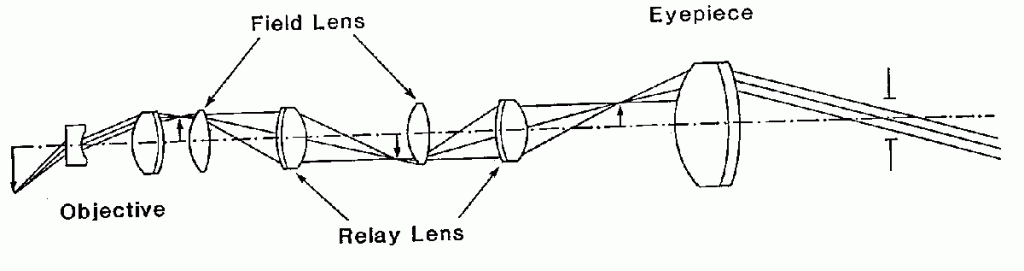
Figure 3. Optical layout of a rigid endoscope
5. RIGID ENDOSCOPE OPTICS
The rigid endoscope optical design is similar to that of a periscope. With reference to Figure 3, the objective lens system forms an inverted image of the internal organ to be observed. A field lens placed near the image redirects the chief ray towards the center of the relay lens system. Another field lens keeps the chief ray confined to the small diameter of the endoscope needle. This succession of relay lenses and field lenses is repeated as necessary for the required insertion depth of the instrument. A conventional ocular magnified the image.
5.1 Relay Optics
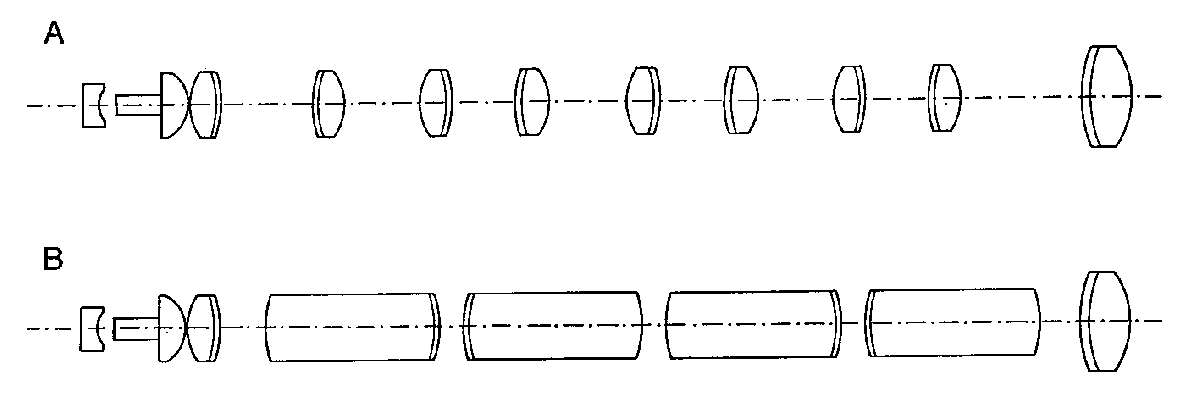
The traditional endoscope employs a series of achromatic doublets for the relay optics (Figure 4a). Modern endoscopes more typically utilize much longer lenses (Figure 4b), with length-to-diameter ratios as high as 10. These are the so-called rod lenses of the Hopkins design6. The advantage of the rod lens designs is that the chief ray bundle can be confined more tightly to the optical axis by reducing the ray divergence in the air gaps between the lenses. This reduces vignetting, one of the most severe problems in small diameter rigid endoscopes. The image also forms further down the endoscope tube, which reduces the number of air-to-glass interfaces in the system.
The mounting of rod lenses is also simpler than thin achromats. The rod lenses tend to tilt less when they are assembled into the inner lens tube. Also, since the spacers are shorter in a rod lens design, they can be made thinner which increases the clear aperture. Rod lenses are typically fine ground on their outer surface to reduce stray reflection. There are now many variations of the rod lens concept, including a design that combines achromatic lenses with long plane-parallel windows.
Figure 4. Rigid endoscope relay lenses
5.2 Objective Lens
The objective lens system increases the endoscope field of view and is designed to be telecentric in image space to allow connection with the relay system. A typical endoscope objective is shown in Figure 5. The relay section determines the optical invariant of the system so for a given field of view the entrance pupil diameter is determined.
Pupil position is adjusted according to the direction-of-view prism design, denoted by its optical tunnel in Figure 5.
Figure 5. Typical optical layout of a rigid endoscope objective
Fortunately, with the typically large field angles in modern endoscopes, the entrance pupil diameter is very small. This allows the unobstructed passage of the entire ray bundle through the multiple reflection prisms typically used. The plano-concave field-widening lens at the tip of the endoscope reduces the inclination of the chief ray to permit its passage through the prism.
6. ENDOSCOPE PRISM
For many medical procedures, the area of interest to the physician is inclined from the axis of the endoscope. In these cases a small prism at the tip of the endoscope re-directs the field of view to the side. An advantage to inclining the field of view is that the physician can increase the effective field of view simply by rotating an endoscope equipped with a fore-oblique distal prism. As long as the incline is no more than half of the field of view, objects along the axis of the endoscope will always be visible. Prism manufacture represents the most severe challenge to the small endoscope optician.
Figure 6. Some endoscope prisms used for inclining the field of view (a) Refracting prism (b) “Super-incline” refracting prism (c) Single component
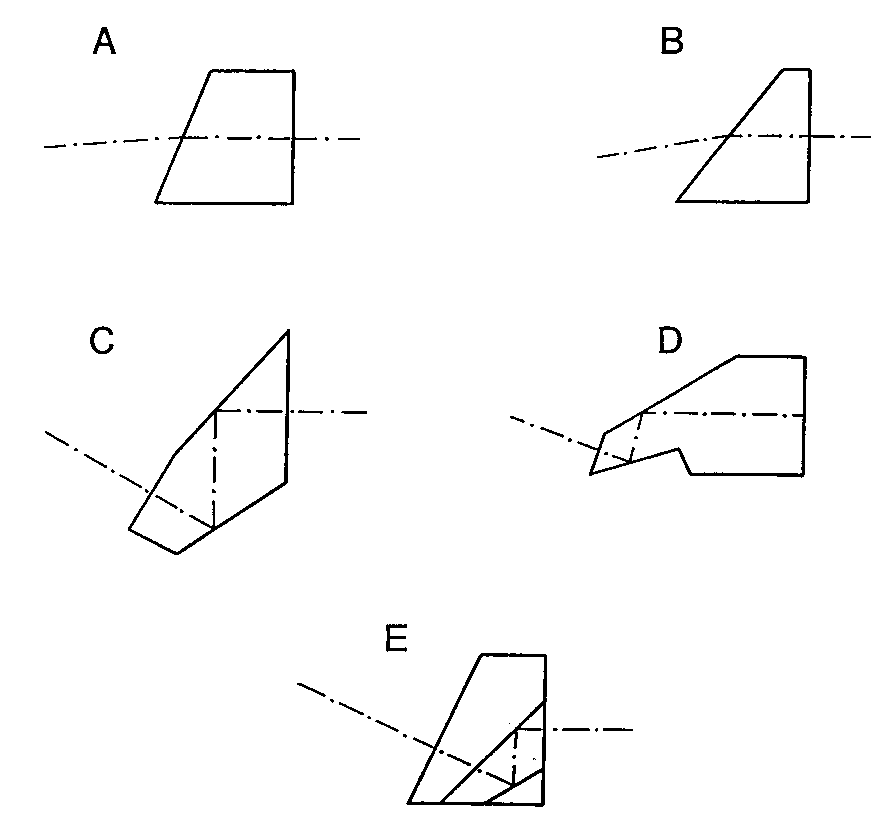
6.1 Refracting Prisms
Figure 6 illustrates several direction-of-view prisms that are currently used in commercial endoscopes. Figures 6a and 6b are cylindrical cross-section refracting prisms and are the simplest to manufacture and the least effective. These prisms are typically epoxied to the end of the objective lens system as a thick window and are ground and polished at an angle after the endoscope needle has been assembled. The limit of incline is dictated by the angle at which the edge of the field of view begins to intersect the tip of the prism. The prism in Figure 6a depicts the maximum prism wedge for commercially available Selfoc endoscopes that are usable in air. Since endoscopes are invariably used with the tip of the endoscope immersed in saline, the direction of view of this prism is reduced to only about 5°. In many instances it is not necessary for the endoscope to be usable in air and the prism wedge angle can be increased to the maximum usable in saline. This produces an incline of the center of the apparent field of view of about 10° for a Selfoc endoscope. Aberrations introduced by these types of refracting prisms are severe, especially distortion and lateral chromatic aberration. Refracting prisms are only used in rigid endoscopes with total outside diameters less than 2 mm where reflecting prisms are extremely difficult and expensive to fabricate. Demands of the marketplace is now requiring the elimination of these ineffective refracting prisms and their replacement with prisms giving a larger field incline with less aberration.
6.2 Reflecting Prisms
Figures 6c-e are various configurations of reflecting prisms which deviate the field-of-view by 30° . Each of these prisms contains two reflecting surfaces and thus retains the proper reversion of the image. Figure 6c is the only prism depicted which does not have an overall cylindrical cross-section and actually hangs down slightly below the mounting envelope of the lens system. This prism must be individually centered to the entrance pupil of the rest of the endoscope optics. Figure 6d is also a one-piece prism but is has the advantage of having a cylindrical cross-section and can be mounted in the same inner tube as the rest of the optical components. The prism shown in Figure 6e is a three-component design with one of the internal surfaces masked and aluminized over only half of its clear aperture. This prism is fabricated from three long wedges that are coated, bonded, and diced. The final step entails edging the prism to the required diameter. These prisms have been successfully fabricated with outer diameters of 1 mm.
7. MECHANICAL CONSIDERATIONS
Fragility of the endoscope needle when used in a clinical environment will always pose a problem.7,8 The tendency of some surgeons to use endoscopes for manipulation aggravates the problem. Rod lenses are often triplet designs with a long central element. When the endoscope needle is slightly bent in use, stresses at the cement joints can separate the lenses. By reducing the diameter of the central element along most of its length except for the ends in the vicinity of the bond lines, the risk of lens separation is reduced.
Gradient-index endoscopes are particularly susceptible to breakage and lens separation because they are typically very thin and have long glass lengths. By cutting the long relay lens into half-period sections and introducing slight spaces between the sections, stresses are reduced in the glass. Cutting the Selfoc at the aperture stops reduces the chance of dirt adversely affecting the image.
7.1 Sterilization
Another consideration unique to medical endoscope design is sterilization. Most surgical instruments are sterilized prior to use in an autoclave, in which a combination of high temperature and pressure kill the bacteria and spores present. Autoclaving an endoscope can be detrimental by two mechanisms. A normal endoscope because of its small size has a low thermal inertia and the inside of the scope will experience high temperatures during sterilization. Normal lens adhesives will separate at these temperatures. Even with high temperature adhesives, small amounts of steam infiltrate the endoscope interior at each autoclave cycle eventually leading to irreversible fogging of the optics. There is much development work being pursued at endoscope manufacturers to produce an autoclavable endoscope although claims of autoclavability seem to be leading true functionality. An endoscope design that can consistently survive more than 100 autoclave cycles would be welcome in the hospital environment.
Until a truly autoclavable endoscope is perfected, hospitals have been forced to use two less convenient regimens. The first method is to expose the endoscope to the poisonous environment of ethylene oxide. This is very effective and does not stress the endoscope optics. Unfortunately, the endoscope cannot be used the same day as the sterilization but must be isolated until the gas residue dissipates. This means that the hospital must stock large numbers of backup endoscopes, an expensive proposition.
The most common means of preparing an endoscope for surgery is by disinfection in a solution of activated glutaraldehyde. For the disinfection times typically used in hospitals spores are not killed, so this regimen is not strictly a sterilization. Infection rates are extremely low after endoscopies and most hospitals allow disinfection by soaking. There are still hospitals, however that prohibits anything other than a true sterilization by gas or autoclave.
8. CONCLUSION
Developments in both flexible and rigid endoscopy have centered on reducing the diameter of the part of the endoscope that is inserted into the body. With flexible endoscopes, applications are being pursued to image the interiors of arteries for visualizing potential targets for laser angioplasty. Gradient-index objective lenses with diameters down to 0.25 mm are commercially available for these applications.
Solid-state video devices that can provide excellent resolution in the same package size are replacing larger diameter flexible fiber endoscopes. These video chips provide color imaging and can be made arbitrarily long. If these video devices can be reduced in size to less than 3 mm, they could even be used in place of some rigid endoscope relay lens systems.
Rigid endoscope developments have concentrated on orthopedic designs that can be used in new areas of the body such as the temporomandibular joint (jaw) and the small joints of the ankle and wrist. If the successes that have been achieved with knee arthroscopes can be duplicated in other parts of the body, we will all benefit from new procedures that are more cost effective, less traumatic, and safer than current techniques.
9. REFERENCES
- L. L. Johnson, Diagnostic and Surgical Arthroscopy, C.V. Mosby, St. Louis (1981).
- G. Berci, ed., Endoscopy, Prentice-Hall, New York (1976).
- M. Watanabe, Atlas of Arthroscopy, Springer-Verlag, New York (1979).
- K. Nisioka, U.S. Patent 4,662,725, “Objective Lens System for Endoscopes,” (1897).
- R. Prescott and D. Leiner, U.S. Patent 4,515,444, “Optical System,” (1985).
- H.H. Hopkins, U.S. Patent 3,257,902, “Optical System Having Cylindrical Rod-like Lenses,” (1966).
- K. Storz, U.S. Patent 4,608,966, “Rod Lens and Endoscope Including the Same,” (1986).
- J. Zobel, U.S. Patent 4,723,843, “Endoscope Optical System,” (1988).